
Due to the complexity of measuring the specific refractive index increment (dŮ/dC), static light scattering molecular weight measurements are often conducted using an estimated dŮ/dC value. When making such an estimate however, it is important to recognize the effect of error in the estimate on the resultant molecular weight and 2nd virial coefficient values calculated from the SLS measurement. The Rayleigh equation utilized for single angle static light scattering measurements can be written in the form shown below, where Ů is the solvent refractive index, Rq is the Rayleigh ratio of scattering intensities, l is the wavelength of the incident light, NA is Avogadroís number, A2 is the 2nd virial coefficient, and C is the analyte weight concentration.

After differentiating with respect to dŮ/dC and rearrangement, the relative errors in the resultant molecular weight and 2nd virial coefficient values can be expressed as:
![]()
![]()
As evident in the above expressions, the relative error in the molecular weight and 2nd virial coefficient values is dependent not only upon the error in the dŮ/dC estimate, but also upon the magnitude of the M and A2 values and the concentration range over which the measurements are collected. Figure 1 shows a graphical representation of the influence of dŮ/dC error on the calculated molecular weight and 2nd virial coefficient values for a low molecular weight protein (lysozyme) under near theta solvent conditions (A2 ~ 0). For this specific example, the relative error in MW and A2 is approximately twice the relative error in the dŮ/dC estimate.

Figure 1: Relative error in molecular weight and 2nd virial coefficient as a function of error in the dŮ/dC estimate for lysozyme under near theta solvent conditions.
The specific refractive index increment is a property associated with a given particle-solvent pair, under a specific set of conditions. It is dependent upon the temperature, the wavelength of the light used in the measurement, the conformation of the particle, and the presence of additives in the dispersant. Ideally, dŮ/dC should be measured under the appropriate solution conditions and at the specified wavelength, prior to beginning any static light scattering experiment. In the absence of the ideal, then yes, dŮ/dC can be estimated, but one should take the time to insure that the estimate is reasonable. For most static light scattering samples, the dŮ/dC value will fall within a range of 0.1 to 0.2 mL/g, with 0.185 being a commonly used value for globular proteins in aqueous buffer and 0.1 being a commonly used value for linear polymers in organic solvents. Since this range amounts to 100% variation, it is instructive to examine the influence of various parameters on the dŮ/dC value of the analyte-solvent pair.
Wavelength Effects
The wavelength dependence of dŮ/dC is expected to follow the Cauchy relationship, with dŮ/dC being proportional to l-2. Figure 2 shows an example of this l-2 dependence of dŮ/dC for a series of globular proteins in water. Note that the dŮ/dC values in this and subsequent figures within this document are extracted from Chapter 6 of Light Scattering From Polymer Solutions (Ed. M.B. Huglin; Academic Press: New York, 1972).
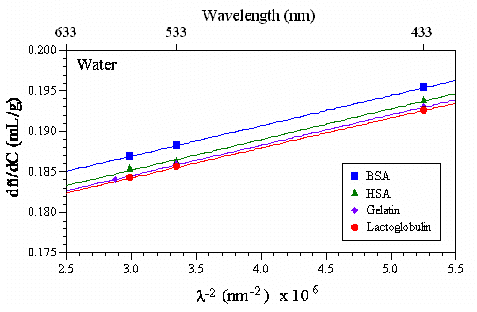
Figure 2: Influence of wavelength on dŮ/dC for a series of globular proteins in water.
Of particular interest regarding the above figure, is the observation that the slope [D(dŮ/dC)/Dl-2] is independent of protein type. As discussed in the following section, this observation should not be too surprising, in that the refractive index (Ů) is strongly dependent upon the partial specific volume (inverse density), which tends to be relatively constant for globular proteins.
The data in Figure 2 also helps to justify the 0.185 rule of thumb value for dŮ/dC for globular proteins. The standard laser wavelengths for light scattering instruments are 633 and 532 nm. For this wavelength range, the range of dŮ/dC values for the globular proteins shown in Figure 2 is 0.182 to 0.188, or better stated as 0.185 Ī 1.5%.
Conformation Effects
As noted earlier, the independence of D(dŮ/dC)/Dl-2 on protein type is likely a consequence of the globular nature of proteins. The refractive index of a solution is a function of the solution density. For globular proteins, the specific volume (1/r) is relatively independent of protein type (vsp = 0.73 cm3/g). Hence the similarity between the dŮ/dC and D(dŮ/dC)/Dl-2 values for the globular proteins examined here. As shown in Figure 3 however, significant changes in both dŮ/dC and D(dŮ/dC)/Dl-2 are observed when the macromolecules are coiled, rather than globular. With regard to error, if one were to use the 0.185 rule of thumb value for proteins for a static light scattering measurement of Dextran, the ~25% error in dŮ/dC could propagate into very large errors in the calculated molecular weight and 2nd virial coefficient values.

Figure 3: Comparison of dŮ/dC values for Dextran, a random coil polysaccharide, to those of a series of globular proteins.
Additive Effects
Additives can influence dŮ/dC in two ways. First, the incorporation of additives can change the refractive index of the dispersant or medium surrounding the particle of interest; and second, the presence of additives can lead to a change in the conformation of the particle being measured. Examples of the former effect can be seen in Figure 4, which shows the influence of salt on the dŮ/dC values for BSA and lactoglobulin, two globular proteins reported to have stable conformations under the conditions cited. As seen in this figure, the addition of salt leads to a decrease in dŮ/dC, but has little to no influence on the wavelength dependence [D(dŮ/dC)/Dl-2].

Figure 4: Influence of salt on dŮ/dC values for two globular proteins, BSA and lactoglobulin.
The latter effects, i.e. conformational effects, are difficult to deconvolute from dispersant refractive index effects. However, the influence of salt dependent molecular conformation can be inferred from the dŮ/dC data shown in Figure 5, which shows the effects of NaCl concentration on the dŮ/dC values for a globular protein and a linear polyelectrolyte at 25 C and 633 nm wavelength. As salt is added to the PAA solution, charges are shielded and the polymer collapses into a more condensed conformation, with the dŮ/dC value approaching that of a globular protein at higher salt concentrations.

Figure 5: Influence of salt/conformation on dŮ/dC values for poly(acrylic acid) & BSA.
Temperature Effects
As noted throughout this document, both the refractive index (Ů) and specific refractive index increment (dŮ/dC) are closely correlated with the density of the solvent and particle being measured. As such, one would also expect to see a distinct temperature dependence in dŮ/dC values. This dependence is exemplified in Figure 6, which shows the influence of temperature on the dŮ/dC values for the series of globular proteins examined earlier. The similarity in the D(dŮ/dC)/DT trends is a direct consequence of the similarity in the partial specific volumes (1/r) of the proteins.
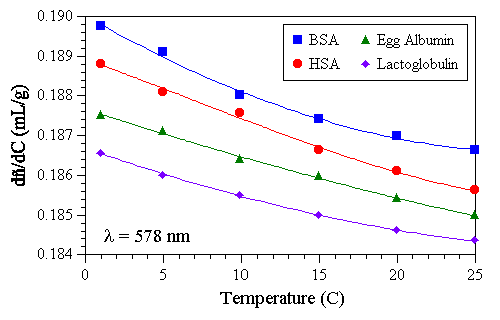
Figure 6: Influence of temperature on the dŮ/dC values for a series of globular proteins in water at a wavelength of 578 nm.
Molecular Weight Effects
If the analyte being measured is a linear polymer, molecular weight is another factor that can influence the dŮ/dC value. Figure 7 shows the molecular weight dependence of the dŮ/dC value for polystyrene in toluene at 25 C and 633 nm. For MW > 1.7 kDa, the refractive index increment values are consistent with the rule of thumb of 0.1 for linear polymers in organic solvents. But for MW < 1.7 kDa, the dŮ/dC values drop by ~20% to 0.8 mL/g.

Figure 7: Molecular weight dependence of the dŮ/dC value for polystyrene in toluene at 25 C and 633 nm.
For additional questions or information regarding Malvern Instruments complete line of particle and materials characterization products, visit us at www.malvern.com.
